Explain How 4p Orbitals Are Different From 3p Orbitals.
So the 3 4 p. Electrons fill low energy orbitals closer to the nucleus before they fill higher energy ones.
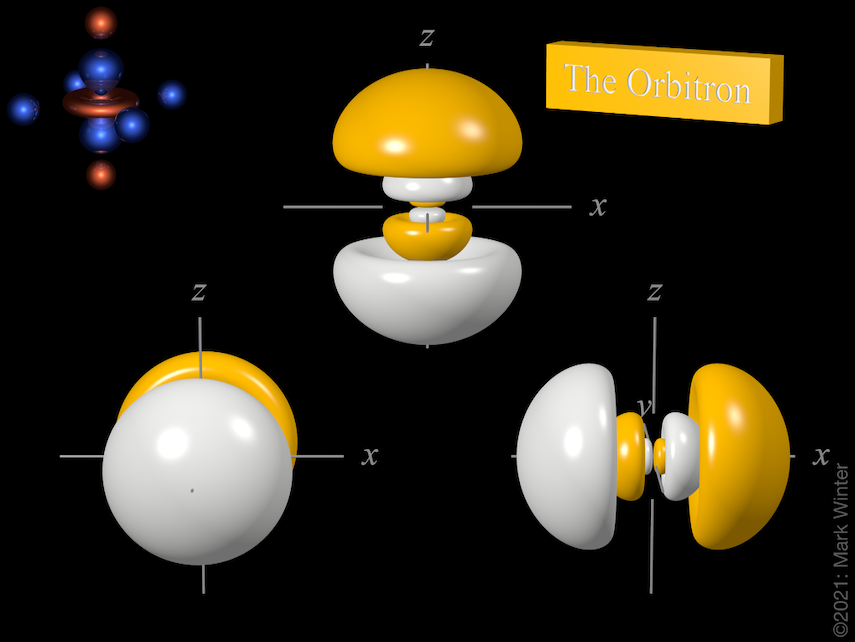
The Orbitron 4p Atomic Orbitals
So this explains why even though we fill the 4s before 3d orbitals we will still ionize 4s electrons before 3d electrons.

. 4p orbitals are larger in size than 3p orbitals and contain no nodes. The order of filling orbitals. Now come towards your question as you ask about difference between 2p and 3p orbital the answer is that 3p orbital has same structureshape that of 2p orbital has but larger in size and energybecause it lies in 3rd orbit of an atom similarly 4p orbitals will have same shape but higher energy and larger in size but shape will be similarsimilarly 5p orbital and.
By the Aufbau principle 3p will be filled first before 4p. Similar to s orbitals size and energy of p orbitals increases with an increase in the principal quantum number 4p 3p 2p. The p sublevels are named 2p 3p and 4p since the p sublevel appears only starting the 2nd level.
It is convenient to define an x y and z axis system and then label each p orbital as px py and pz. Advertisement Answer 0 06gavinlim. Orbiters process the same or different values of a principal quantum number.
Once 3d orbitals are occupied by electrons like in the case of transition elements because they are closer to the nucleus they will repel the 4s electrons further away from the nucleus and cause it to have higher energy level. Now come towards your question as you ask about difference between 2p and 3p orbital the answer is that 3p orbital has same structureshape that of 2p orbital has but larger in size and energybecause it lies in 3rd orbit of an atom similarly 4p orbitals will have same shape but higher energy and larger in size but shape will be similarsimilarly 5p orbital and. This is because of the energy present on the level.
Thus a 2p orbital has 1 node and a 3p orbital has 2 nodes. The 3p 4p 5p and higher p orbitals are all similar in shape to the 2p orbitals but they contain additional nodes as same as higher s orbitals and are progressively. Contain Explain have 5p orbitals are different from up orbitel Al 5p orbitals lorgoy in size then 40 obi tals and contain oolelitional nodes no rodes conturn len rodes D ap orbital ore forger in size then 5p orbitals and contain additirab nder orbitals are smaller in size them up orbitals and contain additional noctes 315p.
The diagram not to scale summarises the energies of. Nodes can be either angular or radial. Both s orbitals and p orbitals are atomic orbitals.
Hence we can say that there are five d-orbitals. Thus we can say that there are three p orbitals whose axes are mutually perpendicular. The main difference between s orbital and p orbital is that s orbitals are spherical shaped whereas p orbitals are dumbbell shaped.
Explain how 4p orbitals are different from 3p orbitals. The Shape of d Orbitals. 9 What is the shape of a 3p orbital.
This means in total the 4p orbital can hold 6 electrons all of which are located in the 4th shell. The 3p orbitals have the same general shape and are larger than 2p orbitals but they differ in the number of nodes. 4p orbitals are larger in size than 3p orbitals and contain less nodes.
Chemistry questions and answers. So the principle quantum numbers harder for. These orbitals indicate the most probable region where we can find an electron of that atom.
O 3p orbitals are larger in size than 4p orbitals and contain additional nodes. The P shell can have 3 orbitals each holding 2 electrons and are orientated perpendicular to each other along each axis as seen below. 4p orbitals are larger in size than 3p orbitals and contain additional nodes.
In this question we have touched that. Up to 24 cash back The three p orbitals differ only in their orientation and are orthogonal to one another. Okay so the principle quantum numbers are the same.
However the energy of an electron in multi-electron atoms depends on both on its principal quantum number n and its azimuthal quantum number l. You have probably noticed that the total number of nodes in an orbital is equal to n 1 where n is the principal quantum number. The magnetic orbital quantum number for d orbitals is given as -2-10 12.
Orbiters are four p. Orbitals can be ranked in the increasing order of orbital energy as follows. Each of the p sublevel has 3 orbitals allowing them to contain 6 electrons as each orbital may hold two.
8 What is the difference in the orbital angular momentum of 2p and 3p electron explain. Do the 34 P. 10 How would the 2s 2 S and 3p.
Where there is a choice between orbitals of equal energy they fill the orbitals singly as far as possible. So the house such a hair is same. Four py and four P.
1s 2s 2p 3s 3p 3d 4p 4d 4f.

Quantum Numbers N L M S Describe The Properties Of An Atom S Electron Configuration Each Electron In An Atom Can Be Described Completely By These N Quimica

Solved Explain How 4p Orbitals Are Dilierent From 3p Orbilals 4p Orbitals Are Larger In Size Han 3p Orbitals And Contain Less Nodes 4p Orbilals Are Larger In Size Than 3p Orbitals And

Solved Explain How 4p Orbitals Are Different From 3p Chegg Com
Comments
Post a Comment